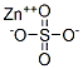Zinc sulphate / CAS:7733-02-0
specification
| Item | Specification |
| Appearance | White or slightly yellow crystalline powder |
| content | ≥92.0% |
| Content of insoluble matter | ≤0.1% |
| Lead content | ≤0.01% |
Usage
Zinc sulfate, with the chemical formula ZnSO4, is a colorless rhombic crystal with a relative density of 3.54. It decomposes into zinc oxide when heated to 770℃. The hydrate is zinc sulfate monohydrate ZnSO4·H2O, a white powdery crystal with a relative density of 3.31. When heated to 280℃, it loses water and becomes anhydrous. Zinc sulfate hexahydrate ZnSO4·6H2O is a colorless monoclinic or tetragonal crystal with a relative density of 2.07. When heated to 70℃, it loses 5 molecules of water of crystallization to become zinc sulfate monohydrate. Zinc sulfate heptahydrate, ZnSO4·7H2O, commonly known as "alum" or "zinc alum", is the most common type. It is a colorless rhombic crystal or white crystal powder. It gradually efflores in dry air, with a relative density of 1.96. At 39℃, it loses one molecule of crystalline water to become zinc sulfate hexahydrate. Zinc sulfate is highly soluble in water, and its aqueous solution is weakly acidic. It can dissolve in glycerol and is slightly soluble in ethanol. It forms a double salt of the ZnSO4·K2SO4·6H2O type with potassium sulfate. When metallic zinc or zinc oxide is dissolved in dilute sulfuric acid and concentrated and crystallized at 30℃, heptahydrate zinc sulfate can be obtained. Then, by raising the temperature, various other types of zinc sulfate can be obtained. It is used as a mordant in dyeing and printing, a wood preservative, a bleaching agent in papermaking, and is also applied in medicine, artificial fibers, electrolysis, electroplating, pesticides and the production of zinc salts, etc
zinc sulfate ZnSO4, also known as alum or zinc alum, is a colorless or white orthorhombic crystal or powder at room temperature. It has astringent properties, is highly soluble in water, and its aqueous solution is acidic. It is slightly soluble in ethanol and glycerol. Pure zinc sulfate does not turn yellow when stored in the air for a long time. When placed in dry air, it loses water and becomes white powder. There are various hydrates: the stable hydrate in equilibrium with water within the range of 0-39℃ is zinc sulfate heptahydrate; within 39-60℃, it is zinc sulfate hexahydrate; and within 60-100℃, it is zinc sulfate monohydrate. When heated to 280℃, all hydrates completely lose their crystalline water. At 680℃, they decompose into zinc oxysulfate. Above 750℃, they further decompose. Finally, at around 930℃, they decompose into zinc oxide and sulfur trioxide. ZnSO4·7H2O and MSO4·7H2O (M = Mg, Fe, Mn, Co, Ni) form mixed crystals within a certain range.
Packaging and Shipping
25KG/Drum or as customer requirements.
Belongs to common goods and can deliver by ocean and air
Keep and storage
Shelf life:24 month from date of manufacture in original unopened packaging stored in a cool dry place out of direct sunlight, water.
Ventilated warehouse, Low temperature drying, Separated from oxidants, acids.