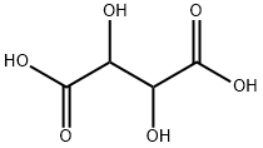
D(-)-Tartaric acid/ CAS:526-83-0
specification
| Item | Specification
|
| DL-tartaric acid content (calculated on dry basis) / (%) is high | 99.5 |
| Melting point range / (℃) | 200-206 |
| Sulfate (calculated as S0) / (%) | 0.04 |
| easily oxidizable substances | conforms |
| Loss on drying/(%) | 0.5 |
| Residue on ignition / (%) | 0.10 |
Usage
Introduction: Because the tartaric acid molecule contains two identical asymmetric carbon atoms, it has three optical isomers, namely dextrorotatory tartaric acid [L(+) -tartaric acid], levorotatory tartaric acid [D(-) -tartaric acid] and racemic tartaric acid (Meso). Equal amounts of L-tartaric acid and D-tartaric acid are mixed to obtain racemic tartaric acid or DL-tartaric acid. L-tartaric acid and d-tartaric acid are enantiomers. All naturally occurring tartaric acid is dextrorotatory. It is colorless transparent crystals or white fine to granular crystalline powder. The crystalline product contains one molecule of crystalline water, is odorless, stable in air, has a sour taste, with a sourness threshold of 0.0025%, and the sourness intensity is approximately 1.2 to 1.3 times that of citric acid. It has a slightly astringent taste and gives off a stench similar to roasted sugar when burned. It also has chelating effects on metal ions. 1g is approximately dissolved in 0.8mL of water (at 25℃),0.5mL of boiling water, and 3mL of ethanol. The pH of a 0.3% aqueous solution is 2.4.
tartaric acid is a carboxylic acid that exists in many plants, such as grapes and tamarind, and is also one of the main organic acids in wine. Tartaric acid, as an antioxidant added to food, can give food a sour taste. Tartaric acid is similar to citric acid and can be used in the food industry, such as in the production of beverages. Tartaric acid, when used in combination with tannin, can serve as a mordant for acid dyes. Tartaric acid can complex with various metal ions and can be used as a cleaning agent and polishing agent for metal surfaces.
Tartaric acid, also known as 2, 3-dihydroxysuccinic acid, dihydroxysuccinic acid, and gluconic acid, has three optical isomers due to the presence of two identical asymmetric carbon atoms in its molecule. Tartaric acid includes D-tartaric acid, L-tartaric acid, and DL-tartaric acid. Tartaric acid is a colorless, transparent prismatic crystal or powder with a strong sour taste and a slight flavor. It is soluble in water and is widely present in various plants in nature in the form of potassium or calcium salts, with grapes having a relatively high content. Tartaric acid is produced from the yellow crystals deposited at the bottom of the barrels during wine-making, hence the name tartaric acid. Its sourness is about 1.3 times that of citric acid. Its uses are similar to those of citric acid. It is highly soluble in water, so it is suitable for making foaming beverages. Tartaric acid crystals contain one molecule of crystalline water, are odorless, stable in air, have a sour taste, with a sourness threshold of 0.0025%, and the sourness intensity is about 1.2 to 1.3 times that of citric acid. They have a slightly astringent taste and give off a stench similar to roasted sugar when burned. They also have chelating effects on metal ions. 1g is approximately dissolved in 0.8mL of water (at 25℃), 0.5mL of boiling water, and 3mL of ethanol. The pH of a 0.3% aqueous solution is 2.4. Dl-tartaric acid is widely used as a food additive, vitamin C effervescent tablets, and a salt-forming acidifying agent (choline tartrate) for organic alkali drugs, etc. Tartaric acid can form acid salts and neutral salts with bases. For example, neutralize it with potassium hydroxide to form potassium hydrogen tartrate. The latter can also be further processed into potassium antimony tartrate (i.e., * tartrate) and sodium potassium tartrate. It can also be used to produce salts such as sodium hydrogen tartrate, sodium tartrate and ammonium tartrate. According to GB2760-86 of our country, tartaric acid can be used as an acidulant in food additives, and is applied in cold drinks, jams, beverages, jelly, canned foods and candies. Due to its low hygroscopicity and good stability, it is particularly suitable for fruit juice and solid beverages. It is also used as a food emulsifier and chelating agent, as well as an antioxidant enhancer. Metal surface cleaners and polishes; Pharmaceutical raw materials, mordants, etc. It is often used in combination with citric acid, malic acid, etc. The limit in sweet foods is 2%(Japan) and 4%(the United States). Precautions for use: This product has a strong sour taste and is slightly astringent. It is often used in combination with other acids. It is mostly used for preparing beverages and other foods with grape flavor. Scope of application and dosage: It can be used in various foods in appropriate amounts as needed for production. Practical usage reference: When used for jams and jellies, it is advisable to maintain the pH of the product at 2.8 to 3.5. It is often used in combination with citric acid, malic acid, etc. This product can also be used in appropriate amounts in candies and baked goods, etc.
Packaging and Shipping
25KG/Drum or as customer requirements.
Belongs to common goods and can deliver by ocean and air
Keep and storage
Shelf life:24 month from date of manufacture in original unopened packaging stored in a cool dry place out of direct sunlight, water.
Ventilated warehouse, Low temperature drying, Separated from oxidants, acids.